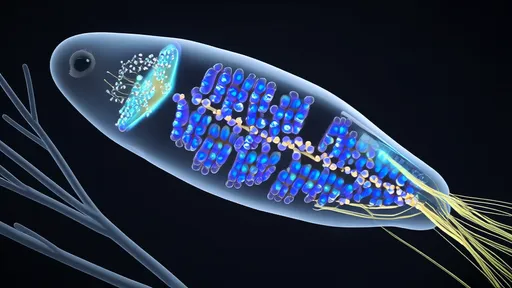
The microscopic world holds countless marvels of biological engineering, and few are as mesmerizing as the coordinated movement of Paramecium—the humble slipper-shaped protist known as the paramecium. Its graceful gliding through water, powered by thousands of tiny hair-like structures called cilia, is a masterpiece of hydrodynamic efficiency. Scientists have long been fascinated by how these microscopic oars beat in perfect harmony, propelling the organism while simultaneously directing food toward its oral groove. Recent advances in fluid dynamics and high-speed microscopy are now revealing the intricate physics behind this ballet of ciliary motion.
At first glance, the paramecium appears to move with effortless simplicity, but beneath its pellicle lies a complex system of ciliary coordination. Each cilium, measuring about 10-12 micrometers in length, beats in a whip-like motion—a power stroke followed by a recovery stroke. What’s remarkable is not just the movement of individual cilia, but their collective behavior. Unlike the chaotic flailing one might expect from thousands of independent filaments, paramecia exhibit metachronal waves—a sequential, wave-like pattern of ciliary beating that propagates along the cell’s surface. This synchronization minimizes drag and maximizes thrust, allowing the organism to achieve speeds of up to 1 millimeter per second, an impressive feat for a creature just 0.3 millimeters long.
The fluid dynamics governing this motion are nothing short of extraordinary. Researchers using micro-particle image velocimetry (μPIV) have mapped the vortices created by ciliary beating, revealing how paramecia manipulate water flow at low Reynolds numbers—a regime where viscosity dominates over inertia. In this microscopic environment, water behaves more like honey than the familiar fluid we experience, making efficient locomotion a significant challenge. Yet, the paramecium’s cilia generate asymmetric strokes: a stiff, forceful power stroke that propels water backward (and the cell forward), followed by a flexible, curved recovery stroke that reduces backward drag. This asymmetry is crucial for net movement in a viscous world where every action has an immediate and equal reaction.
Equally fascinating is how cilia around the oral groove create vortices to sweep bacteria and organic particles into the paramecium’s "mouth." High-speed videos show that these feeding currents are not passive but actively tuned—the angle and frequency of ciliary beats adjust based on food concentration. When nutrients are abundant, cilia near the oral groove beat more vigorously, creating stronger inflow currents. This dynamic responsiveness suggests a level of hydrodynamic sensing that blurs the line between simple reflexes and primitive decision-making.
New studies are challenging old assumptions about ciliary coordination. While it was once thought that the cell membrane’s electrical signals solely synchronized cilia, emerging evidence points to mechanical coupling through the fluid itself. As one cilium beats, it alters the local fluid environment, influencing the motion of neighboring cilia. This hydrodynamic coupling creates self-organizing metachronal waves without centralized control—a phenomenon seen in other ciliated organisms like Platynereis larvae. The implications extend beyond biology; engineers studying micro-robotic swimmers are borrowing these principles to design artificial ciliary systems for targeted drug delivery.
Yet mysteries remain. How do paramecia achieve such precise control despite having no nervous system? Some researchers propose that the infraciliary lattice—a cytoskeletal network beneath the cilia—acts as a mechanical conductor, transmitting tension to coordinate beats. Others speculate that bioelectrical gradients, modulated by calcium ion fluxes, provide timing cues. What’s clear is that evolution has crafted a propulsion system where fluid dynamics, structural flexibility, and biochemical signaling intertwine seamlessly.
The study of paramecium locomotion isn’t just academic curiosity; it’s inspiring breakthroughs in fields from medicine to nanotechnology. Synthetic cilia arrays, modeled after these protists, could revolutionize microfluidics by enabling precise control of fluids in lab-on-a-chip devices. Meanwhile, understanding how cilia avoid entanglement or damage during rapid beating may inform treatments for human ciliopathies—diseases like primary ciliary dyskinesia. Even robotics stands to benefit, with soft robots potentially employing "artificial metachrony" for efficient movement in viscous environments like blood or mucus.
As imaging techniques reach nanometer-scale resolution, we’re witnessing a new era in protist biomechanics. Recent 3D reconstructions using cryo-electron tomography reveal how dynein motor proteins within cilia generate power strokes by "walking" along microtubules. These molecular-scale insights, combined with macro-scale hydrodynamic models, are painting the most complete picture yet of how nature’s simplest swimmers mastered fluid dynamics eons before human engineers even existed. The paramecium, once a mere footnote in biology textbooks, now stands as a testament to the sophistication of evolutionary design—where thousands of tiny hairs dance to the silent rhythm of physics, and every beat tells a story written in water.

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025
By /Jul 7, 2025

By /Jul 7, 2025
By /Jul 7, 2025
By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025
By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025